Implementing the IEC 60601-1 Medical Electrical Equipment Standard
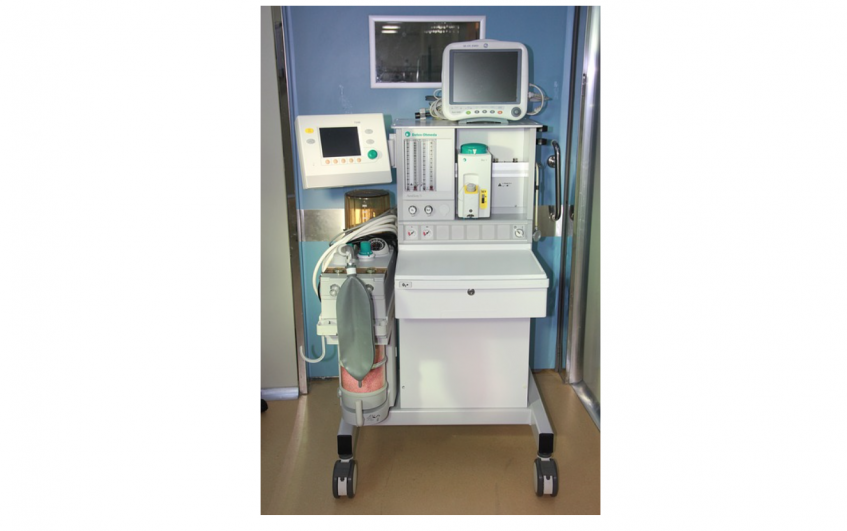
Mobility is a big factor in the mechanical design of a medical device. On the surface are the big obvious questions: Will it move? Does it have wheels? Does it have to be small, light, and portable? But for medical devices, it’s a lot more than that. Under the IEC 60601-1 Medical Electrical Equipment standard, the mobility classification of a device defines which tests need to be conducted in order to evaluate safety in reasonably foreseeable use scenarios. That means incorrectly defining device mobility (or failing to define it at all) may not only result in a different product than you had envisioned, but it may also result in a design that is not suitable for some of the most rigorous mechanical tests that will be required. Failing to plan for this means your device may require major re-designs right before you thought you were ready for regulatory submission and transfer to manufacturing.
For example, we once changed a device from a mobile cart design to a portable design, as the bottom half of the cart design was mostly empty and users had ready access to benchtops. The device could essentially be sliced in half, and the bottom discarded. The remaining top half became a portable unit. Because the portable version had to pass drop testing, have strong handles, and survive impact tests, making the change was not nearly as simple as it seemed on the surface. Luckily, the designers were aware of the implications and discussed the impacts on the development program early enough to allow for effective planning of the new approach.
Mobility is only one of several categorizations defined as part of 60601-1, including (for example) applied parts and patient supports. However, mobility definitions are spread throughout Clause 9 and 15 of the standard, and this has been challenging to navigate in my experience. This article endeavours to bring clarity to this critical categorization and give examples of why mobility is so important. This flowchart will guide you through all possible classifications and their associated tests:
Final chart.jpg
What Is 60601-1 Anyway?
IEC 60601-1 is a widely recognized standard for medical device safety. This means adherence is either mandatory or very helpful (depending on the region) to obtain regulatory clearance. Within the standard, tests are defined and must be completed in order to verify the safety and efficacy of the device. Not every test in the regulation is required for every device; they vary depending on which classification of device you’re designing. For example, a device that never touches patient tissue (such as many types of benchtop analyzers) doesn’t need to be tested for biocompatibility, but devices that do (such as a contact lens) are subject to biocompatibility testing (more about biocompatibility here). Similarly, devices that can be moved (are transportable) have different expectations and are tested differently than devices that are stationary. The standard has seven different classifications for each type of mobility plus an eighth, a parent term as shown in the chart above.
Does This Really Impact My Device?
A good medical device design is readily testable, so testing expectations are a major driver of design. Defining those expectations up front means understanding which tests apply to your device. As mentioned, there are a number of classifications within 60601-1, but mobility can be argued to have the most impact on system architecture, as mobility-related tests affect nearly everything in the device for the reasons described below.
How Much of the Design Is Related to Mobility Classification?
A lot! Almost all mobility classifications are subject to some sort of impact or drop test. The need to survive these tests is a major design driver. Components jarred during impact can have damaging consequences for the enclosure and internal components alike. Therefore, if the mobility is misclassified, a considerable redesign (along with additional cost and an extended schedule) may be incurred to meet the appropriate requirements. Obviously, this could be a major speedbump on the already rigorous path to commercialization.
What Are the Classifications and Which Designs Are Typical of Each?
The sections below describe each classification noted in the chart above and the typical design approaches used to pass the most burdensome mobility tests from each classification. As you can see, the differences between some of these classifications are fairly minimal, but the tests vary for each and are rigidly prescribed. The capital letters in the following excerpts refer to terms that have definitions within 60601-1. As a reminder, this article covers certain mobility tests only—there are numerous other tests applicable to each classification.
STATIONARY: A term referring to equipment that, once installed and placed into service, is not intended to be moved from one place to another. This includes the following subcategories:
FIXED: A term meaning fastened or otherwise secured at a specific location either permanently or so that it can only be detached by means of a TOOL.
PERMANENTLY INSTALLED: a term meaning electrically connected to the SUPPLY MAINS by means of a permanent connection that can only be detached by the use of a TOOL.
All stationary equipment is either fixed or permanently installed. Mechanical designs may focus on the impact and push tests. The former involves dropping or swinging a steel ball weighing 500 g (~1 pound) onto multiple sides of your device from 1.3 m (~4 ft) as in this video, and the latter is a 250 N (56 lbf) push on all sides.
To address the impact test, panels that can either absorb (through deformation) or resist the impact are typically employed. Sometimes, sensitive components are isolated from the external panels or chassis through impact-dampening materials such as polymer washers.
To resist the push test, the device must either be able to topple over without issue (despite jarring all of its internal components) or be wide enough not to topple under the applied load.
TRANSPORTABLE: A term referring to equipment that, once installed and placed into service, is intended to be moved from one place to another whether or not connected to a supply and without an appreciable restriction of range.
Transportable devices must be either mobile or portable.
MOBILE: A term referring to TRANSPORTABLE equipment that, once installed and placed into service, is intended to be moved from one location to another while supported by its own wheels or equivalent means.
Mobile devices are typically wheeled and often utilize a main chassis on which the casters, handles, and user interface devices are attached and in which the guts of the system are installed. That means changes to the chassis could result in changes to almost all of the subcomponents. The most burdensome tests for mobile medical devices are usually rough handling and angled floors, and the impact and push tests are described in the Stationary section above.
To address the first and second elements of the rough handling test, the device should have a low center of gravity and big enough wheels to navigate up and then down a 40-mm threshold when traveling at 0.8 m/s (1.8 mph).
The third element of the rough handling test is meant to mimic ramming the device into a door frame at 0.8 m/s (1.8 mph). Devices often have a purpose-built point of impact that transfers the majority of forces towards elements that can handle them and away from sensitive components. Very sensitive components may need to be isolated with specialty connections. Impact points are also frequently designed to hide superficial damage as much as possible (e.g., have a textured finish) given door frame type impacts will likely occur multiple times over the life of the device.
PORTABLE: A term referring to TRANSPORTABLE equipment that, once installed and placed into service, is intended to be moved from one location to another while being carried by one or more persons. This includes the following subcategories:
BODY-WORN: A term referring to TRANSPORTABLE equipment whose INTENDED USE includes operation while being worn by a PATIENT or attached to a PATIENT’S clothing.
HAND-HELD: A term referring to equipment that, once installed and placed into service, is intended to be supported by the hand.
Portable devices can be body-worn, hand-held, or neither (in which case they are often called bench-top, though that is not a 60601-1 classification). A benchtop device may have an internal metal frame and external plastic panels, much like a typical mobile device. Hand-held and body-worn devices, however, often utilize a more exoskeleton-like design without any internal supports. The most burdensome test for portable devices is usually the drop test.
To address the drop test, a portable device’s exterior is often designed to absorb energy without critical damage and to isolate sensitive internal components. For example, shock-absorbing pads can isolate and also double as thermal and/or electrical isolators or conductors, depending on the material. For larger benchtop devices, internal components may also be supported by a strong internal frame. Any adhesive should be strong enough to absorb the forces associated with a 1-m drop onto hardwood in multiple orientations.
The Impact of Changes Mid-Design
Changes to the device classification mid-design can lead to significant changes throughout the whole device. For example, contrast a mobile device utilizing a chassis design with a strong central frame and rubber pads to protect sensitive electronics during a door-frame test with a stationary device designed with a thin outer frame and a stack up of electronics to save on part cost. If a mobility classification changes halfway through the design process from stationary to mobile, the stationary device may require a complete chassis re-design, often leading to changes in sub-component configuration, design, or interfacing. This is further aggravated by the fact that mechanical parts also affect thermal and EMC characteristics and may double as the cosmetic exterior, too. Of course, you could design for all the tests independent from the actual mobility classification of your device, but that will probably add unnecessary cost, weight, and size, all of which could negatively impact the business case and device usability .
In Summary…
Device mobility classification is an important element of 60601-1. It can be somewhat confusing to understand which classifications are subsets of other classifications. Each mobility classification has its own challenges with different solutions to each challenge. Confusion around the mobility classification of a medical device could lead to a failure to appropriately design for the relevant testing. This will very likely have massive cost and schedule repercussions. Take care to appropriately understand your device’s mobility classification early on and lay a solid foundation for a stable development program.
SOURCE:MDDI










