Ariste Medical co-founder sees great potential for drug-coated implants and orthopedics
It’s been more than a decade since Lisa Jennings launched not one but two companies in the Great Recession.
In 2020, she sold CirQuest Labs to MLM Medical Labs, where Jennings serves as chief scientific officer and managing director of U.S. operations.

More recently, her pre-commercial medtech development startup, Ariste Medical, won FDA 510(k) clearance for its antibiotic-coated hernia mesh in March.
You can expect more to come from that technology, Jennings told Medical Design & Outsourcing in an interview covering future applications, the drug-device development process and what she’s learned through it all.
“We hope this encourages thinking about more ways of mitigating complications with implantable devices,” Jennings said. “There are so many possibilities out there, new drugs being developed all the time. It’s really exciting to think that with various coatings out there and the types of devices, the applications for this technology can even be extended as we learn more about disease process, and we learn more about new drug targets and develop new drugs that can mitigate some of these complications.”
MDO: Is there anybody else doing the kind of work you’re doing?
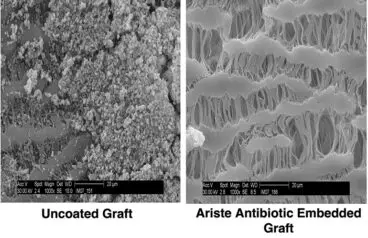
Jennings: This is the first product that we’re aware of that combines the benefit of the polypropylene mesh in addition to a coating that allows residence of antibiotics to mitigate bacterial colonization for surgical repair of soft tissue. There have been some products where devices have been coated with antibiotics. We developed a proprietary coating that is biocompatible and allowed us to incorporate antibiotics into the coating, such that there was a drug residence or a drug-embedded technology, and this can broadly be used not only for other implantable devices, but also for nonimplantable devices. The beauty of this technology is that it is a game-changer for polypropylene mesh used for open ventral hernia repair, but we also have done proof-of-concept testing and demonstrated that our technology can be applicable for a variety of materials such as polyurethane and ePTFE (expanded polytetrafluoroethylene, also known as Gore-Tex). ePTFE is known to be one of the slippery surfaces, but we could also use our coating technology on ePTFE surgical implants such as vascular grafts. And we also have proof of concept that this can be used on metals, so there’s a lot of potential in the orthopedic space.
MDO: What are the common implantables and nonimplantables made from these kinds of materials that you’re eyeing as potential applications?
Jennings: We have proof of concept in vascular grafts that would be used for peripheral bypass — the same material is often used for dialysis access — central venous catheters, and urinary catheters. We’ve done some work in that area already and in some of the orthopedic metals. This could go into several different directions as far as looking at market opportunity: perhaps sutures, shunts, maybe even small-diameter grafts, tissue patches, things of that nature. We think there’s opportunity that can be extended to other products that target complications due to bacterial or other microbial infections or that can target other areas. We have some proof of concept in terms of reducing scar tissue formation with other drugs where these vascular grafts are used. We have very preliminary R&D type data and we’re confident that this particular technology is a platform technology that can be extended to other products.
MDO: And what other drugs are showing potential?
Jennings: In the coronary stent market, metal stents have an antiproliferative incorporated onto their stent to reduce scar tissue formation. We found a candidate drug that has been used in these metal stents for coronary artery disease can also be incorporated into our coating and we can apply that to, for example, EPTFE grafts and reduce scar tissue formation. There’s a lot of candidates out there, approved drugs that have been used in the coronary stent indication that could easily be incorporated into our coating and serve that same purpose for other implant materials.
MDO: It sounds like if you can coat it, you can drug it?
Jennings: We haven’t done an extensive survey of a lot of drugs, but the ones that we have used, we’re seeing the expected effect of the drug, the activity of the drug. I’m a vascular biologist by training, and my area of expertise is in the area of thrombosis. I could see many applications for clotting, for acute thrombosis that may occur when grafts or other catheters are used in patients, and it’s possible that we could mitigate clotting and reduce the extent or the generation of a thrombus during that acute period. We’re definitely interested in the orthopedic space, because there’s a fairly significant incidence of infection in some of the implantable devices in orthopedics. … The platform could be pretty impressive in terms of where we could use our coating, our way of incorporating drugs and preserving their activity. Plus, we took it a step further and developed a methodology for coating devices that basically ensures that the desirable device properties are not compromised, but that we do have a consistent coating for each of the product lines. We’re very excited about our automated coating device that we can program for the appropriate coating cycles that are needed for a particular device.
MDO: Does it spray? Does it dip? How does it apply that coating?
Jennings: It’s more of a controlled motion. We have basically a drive system that allows us to coat the product, and then depending upon the polymer itself, how that polymerization is integrated into the coating process. It’s a controlled coating process that allows for a consistent polymer application to the device. And that’s pretty much all I can say.
MDO: What was the big obstacle or hurdle? Why has nobody done this before?
Jennings: I think it’s just recognition of an unmet need. This technology came out of the University of Tennessee Health Science Center. At the time, Dr. Tim Fabian was the chair of the department of surgery here in Memphis, and I was the director of the Vascular Biology Center of Excellence. We pulled together a few people on campus to help us think about what a coating might look like. We just put our heads together and started doing some R&D at the university. We were fortunate to come up with something that showed promise and we submitted that discovery to our research foundation and a patent was filed. Then Tim and I, along with Brian Best — who’s been a colleague for a number of years and has expertise in commercialization and product development — founded Ariste Medical and decided we would identify a product that we could tackle and hopefully carry it through to 510(k) approval. We were fortunate to get some initial angel investment that allowed us to really open up the company. That was in 2012. We continued R&D, filed some more patents, and by 2015 we felt we were ready to move forward with what the product prototype would look like. We were fortunate to get another round of investment from about 2015, 2016 until approval just this past March. We’ve been doing the studies necessary to submit to the FDA for clearance, and we were obviously very excited to get the news this March.
MDO: How soon might we see FDA approval for some of those other applications?
Jennings: There’s certainly a plan. We’re a small company. We really focused on this one product, and we have over recent years developed that proof-of-concept data to be able to move forward in some other product lines. But we’re just in the process of moving forward on strategies of that nature. Our thinking was we would continue to do R&D, but being a small company, our focus was to get this first product through the FDA.
MDO: What did it take to choose the right material?
Jennings: We did a lot of different R&D, we combed the literature of what was developed over the years in terms of coatings, we brought in our own chemist — we’ve had other consultant chemists work with us — and it was taking that knowledge and the experience of our chemist to develop some prototype coats. We were looking for something that would not compromise the implant material itself. For example, with polypropylene mesh, there’s a certain porosity, there’s a certain weight, there’s a certain tensile strength and a certain flexibility that the physician is used to working with. We did engagement with key opinion leaders, particularly infectious disease and the surgery space. In fact, Dr. Mike Rosen on our board of managers is one of the world’s experts in hernia repair and is a physician investigator in his own right, and we certainly value him as a board member and an advisor over the years. There were a lot of avenues to identify what would be the ideal mesh product, the appropriate mesh to choose from since there’s lots of different properties of mesh, and then the know-how and experience of our chemists. My interest was in biocompatibility, understanding cellular response to materials that are exposed to the tissue. We brought in two top production engineers to help us devise a coating technology that would be acceptable for coating these devices, and then our director of product development, Olaf Schulz, really carried the directive of the lab to do the necessary tests and generate and gather the data that were needed for the submission. It’s just — as a lot of people will agree — it’s having the right team and we pull together engineers and chemists and biologists and brought in experts that would help guide us in terms of what would be the appropriate product that the physician would embrace and in their opinion would hopefully improve the outcome of their patients.
MDO: What have you learned through all this?
Jennings: It’s an interesting journey, that’s for sure. I’m a little bit of an entrepreneur at heart. I formed a laboratory services company around the same time that we formed Ariste Medical, grew that company for 12 years and had an acquisition in July of 2020. I was kind of juggling both Ariste and my lab services company concurrently. There is much to learn and whether it’s product development or lab services, I look back and certainly this company was very organic and we formed the company at the worst possible time during the economic turndown. There wasn’t a lot of investment taking place in 2008, 2009. It took a little while to gain some traction and we were fortunate to have some angel investment. It seems like it’s been a long time coming, but in some ways, having that time and really just looking at what was technically feasible, what was the medical need in these types of technologies, as a small company we couldn’t guarantee what path was in front of us. A 510(k) opportunity is certainly easier for a small company than a PMA and technically feasible. We just looked at all the possibilities because there were many different products we could have gone after and this one seemed to be the best one for us. So we took time to do that. It took time to do the R&D and then we looked at the competitive landscape. Synthetic base meshes have been around for a while. There had been an interest in resorbable meshes, but we were interested in still providing that structural integrity and some defects require the strength of polypropylene mesh. And then there’s the biologic meshes and they’ve been helpful in terms of use in infected and contaminated areas, but they’re really expensive. We think our product is going to have a more palatable price point. In some ways we just checked the boxes, we had talented people in the company and people who saw the unmet need, and all the stars aligned. We were fortunate in developing this. Continuing to touch base with the key opinion leaders and the hernia surgery space served us well, because we wanted the physician to have something they’re comfortable using that had good success in their patients, and yet hopefully provided value-added, perhaps even peace of mind benefit.
MDO: If you could go back and give yourself a message when you were starting all of this, what, what would you tell yourself?
Jennings: The R&D path served us well. Engaging a regulatory consultant sooner for navigating through the submission the approval may have expedited that or shortened the timeline. We did utilize a regulatory consultant, but probably earlier than later is a good idea because they’re certainly helpful and have gone through that process with the FDA and can certainly advise a company in terms of focus and how the FDA will view the data and how much data would be necessary to gain that approval. Having someone with that regulatory expertise would be extremely helpful for companies to engage sooner than they may think they need to.
Article source: medical design & outsourcing










