Manufacturing
-
2024.03.19
The Painfully Slow World of Prototyping Before 3D Printing—#ThrowbackThursday
Read More -
2024.03.09
The Troubleshooter: How to Interpret Plastic Injection Molding Data
This article will identify the data collection points that best identify system failures. It will also recommend approaches to recognizing system failures and explain how to adjust and improve production scores.
Read More -
2024.02.26
Additive Manufacturing Can Be a Superpower, If You Know How to Use It
As with any other type of manufacturing process for medical devices, additive manufacturing (AM) requires a specific and careful approach during the design and production-approval phases. When the proper steps are taken during the early stages of development, AM can serve as a powerful production resource.
Read More -
2024.02.20
Small patients, big design challenges: Pediatric device experts guide engineers on solutions
a panel featuring leading physicians and device innovators discussed the unique complexities and challenges of pediatric device development. Joining Søndergaard was Dr. Byron Holt, chief of pediatric cardiology at the Texas Center for Pediatric and Congenital Heart Disease and so on.
Read More -
2024.02.01
Exploring the Versatility of MEMS Pressure Sensors
MEMS sensors are made of a piezoresistive diaphragm connected to a Wheatstone-bridge circuit
They respond to pressure change and generate a proportional electrical current
Compare MEMS sensors with fiber-optic and fluid-filled sensors
Read More -
2024.01.29
Navigating the complexities of micromoulding for medical devices
Micromoulding is a specialised form of injection moulding that produces extremely small, high-precision parts, often with intricate features and complex geometries. In the medical device industry, Micromoulding has become an indispensable technology due to the trend towards miniaturisation and the increasing complexity of medical devices.

Read More -
2024.01.22
Begin With the End in Mind When Developing Wearable Devices
Dave Liebl, chief commercial & technology officer at Intricon, has one main piece of advice for wearable biosensor manufacturers. “If you think there’s a chance that your device would be a medically approved and recognized data source for making medical diagnoses or deliver therapeutic values, then you should start the development of that product with that in mind,” he said in a recent interview with Design News.
Read More -
2024.01.05
Key considerations for electromagnetic sensors for surgical navigation
This article details the advantages of EMN for surgical navigation and explores considerations for optimizing sensor performance, cost, and manufacturability.

Read More -
2023.12.18
What is the Minimum Acceptable LRV for Medical Packaging?
The package must demonstrate strength, integrity, and microbial barrier properties to maintain the sterile barrier through the intended shelf life. However, it is left to the individual medical device manufacturers to determine how to meet the requirement and create acceptance criteria for their sterile barrier packaging validations.
Read More -
2023.12.15
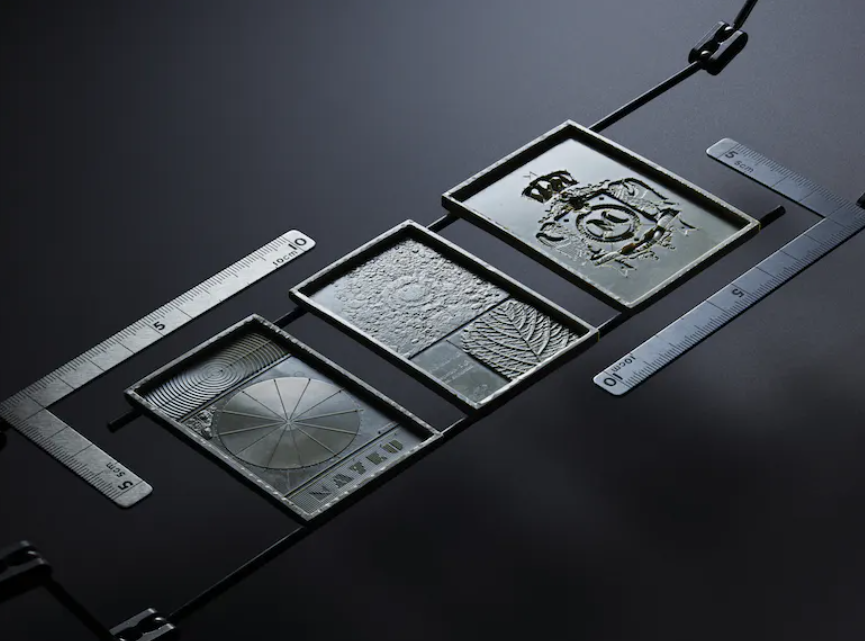
Medical Device Maker Enhances its Packaging 3D Printing Workflow with a Benchtop Multiplier Pressure Former
In the realm of advanced medical device development, Switzerland’s Oertli Instrumente AG has transformed its prototyping and packaging processes by integrating the Mayku Mulitplier pressure former into its 3D printing workflow.
Read More










